
Working Group: Understanding EGAPP
Steps in the EGAPP Working Group Review Process
Download PDF ![]() (460 KB)
(460 KB)
Overview | Step 1 | Step 2 | Step 3 | Step 4

Summary: An evidence review involves many steps and is meant to synthesize available evidence on a particular disorder, test, and clinical scenario.
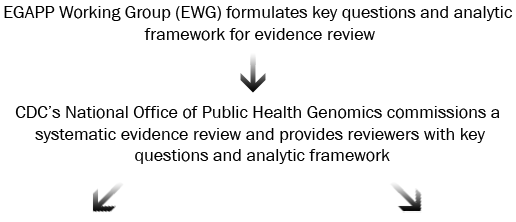
CDC commissions systematic evidence reviews using two strategies:
- Comprehensive Reviews are usually done in partnership with Agency for Healthcare Research and Quality Evidence-based Practice Centers (AHRQ EPCs). EPCs conduct comprehensive literature searches and evaluation, with detailed
documentation of methods and results.
- Targeted and/or Rapid Reviews are conducted for topics with minimal literature to review and/or targeted questions to answer. These reviews are coordinated by CDC-based EGAPP staff in collaboration with technical contractors and expert core consultants.
Evidence reports, the products of these reviews, are detailed, systematic, objective assessments of the available scientific and
clinical evidence on a specific topic. Evidence reports are the basis for deliberations by the EGAPP Working Group as they develop
their Recommendation Statements.
Who is involved: CDC commissions the review, the EWG develops the key questions to be addressed, and the selected review team (e.g., EPC or other contracted group) conducts the review and produces a report. The review team establishes a Technical Expert Panel (TEP) to provide guidance, usually including topic experts and two to three EWG members.
Transparency: All EGAPP Working Group members, review team members, and consultants disclose potential conflicts of interest
for each topic considered. Evidence reports undergo external expert review. Reports or manuscripts published with CDC staff as authors may undergo CDC clearance.
Products:
- Evidence reports posted on web sites (EGAPP or AHRQ)
- Published summaries of evidence from the AHRQ EPCs or other contracted review group.
EGAPP EVIDENCE REVIEW PROCESS
Comprehensive EPC Reviews |
Rapid and/or Targeted Reviews |
|
Conducted by Agency for Healthcare Research and Quality Evidence-based Practice Centers
|
Conducted by reviewers who may include:
|
|
Page last updated: July 19, 2010
Page last reviewed: December 23, 2008
Content Source: EGAPP Team
© 2009 Evaluation of Genomic Applications in Practice and Prevention (EGAPP)
expressed on this web site do not necessarily represent the views of the funding agency, CDC.
Privacy Policy