
Working Group: Understanding EGAPP
Steps in the EGAPP Working Group Review Process
Download PDF ![]() (460 KB)
(460 KB)
Overview | Step 1 | Step 2 | Step 3 | Step 4

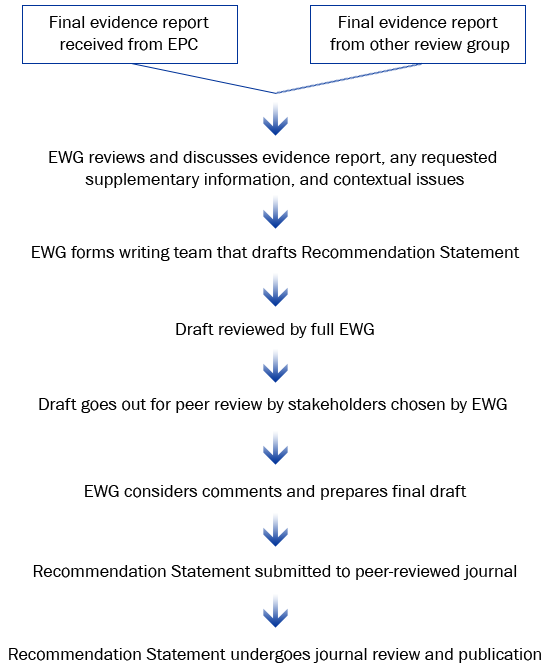
Summary: The EGAPP Working Group (EWG) reviews the evidence report, considers contextual issues, and may consider other
sources of evidence. A draft recommendation statement is developed, peer-reviewed, and submitted for publication.
EWG Recommendation Statements are based on CDC-commissioned evidence reports, other review of evidence as needed, the quality of available data, and potential clinical and social impact of using the test in practice.
Who is involved: EWG, with support from CDC-based EGAPP staff and consultants.
Transparency: Review of comments from industry and a range of stakeholders (e.g., from professional organizations, health plans, consumer groups, and public health programs).
Products:
- Peer-reviewed, published EWG Recommendation Statements
Recommendation Statement Development Process
>> See Step 4: Disseminate EGAPP Products & Communicate Informational Messages to Stakeholders
Page last updated: July 19, 2010
Page last reviewed: December 23, 2008
Content Source: EGAPP Team
© 2009 Evaluation of Genomic Applications in Practice and Prevention (EGAPP)
expressed on this web site do not necessarily represent the views of the funding agency, CDC.
Privacy Policy